Any quality auditor will admit that a set of good inspection records make their job much easier. But what do “good records” actually look like?
José Rodríguez Pérez just published an interesting article entitled ‘Maintaining Data Integrity’ in Quality Progress, the official publication of the ASQ (American Society for Quality).
Data integrity is very important in any quality management system. And it is even more important in highly regulated industries (medical devices, drug manufacturing…).
This article clarifies some of the explanations from the FDA’s guidance document.
What does ‘data integrity’ mean in practical terms?
“Complete, consistent, and accurate data must be attributable, legible, contemporaneously recorded, original or a true copy, and accurate (ALCOA).
Here are definitions of those key attributes:
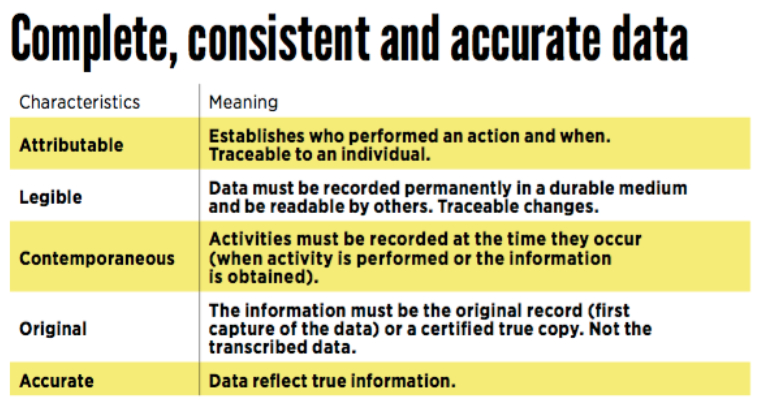
Another very important concept is the audit trail:
“Audit trail means a secure, computer-generated, time-stamped electronic record that allows for reconstruction of the course of events relating to the creation, modification, or deletion of an electronic record. An audit trail is a chronology of the “who, what, when, and why” of a record.
A good audit trail makes it easy to detect red flags such as:
- Backdating information
- Altering original data and records
- Creating acceptable test results without performing tests
- Documenting activities before execution
Your Thoughts…
What are your tips or experiences when keeping inspection records and being audited?
Have I covered everything here, or are there some other record-keeping points and terms that need to be mentioned here?
Please get involved by leaving a comment or question below, and we’ll gladly respond.


